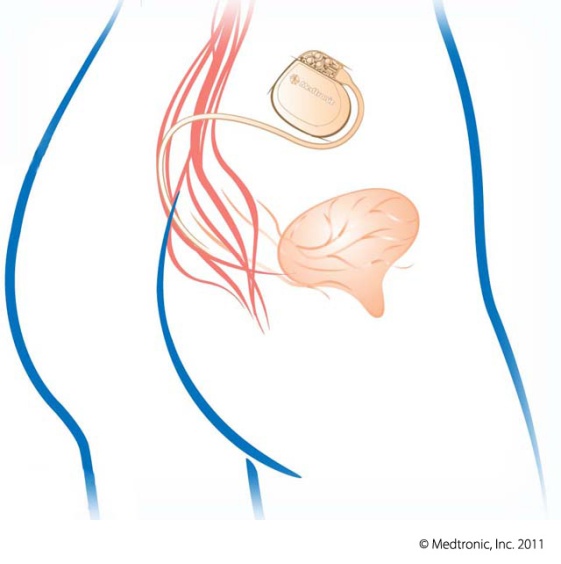
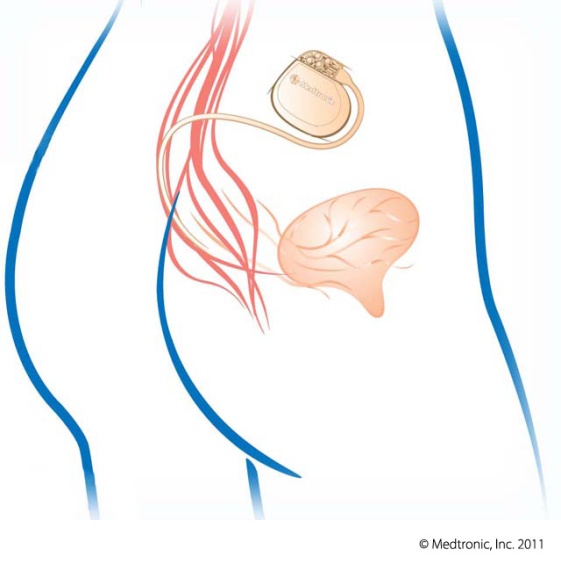
- Performed as an in-office or outpatient procedure
- Using local anesthesia, a thin, flexible wire is inserted near your sacral nerves, connected to a test stimulator worn on your waistband
- Sends mild electrical pulses to the sacral nerve
- Can immediately reduce and even eliminate symptoms of overactive bladder and urinary retention
- Use a symptom log to record how the stimulation affects your symptoms
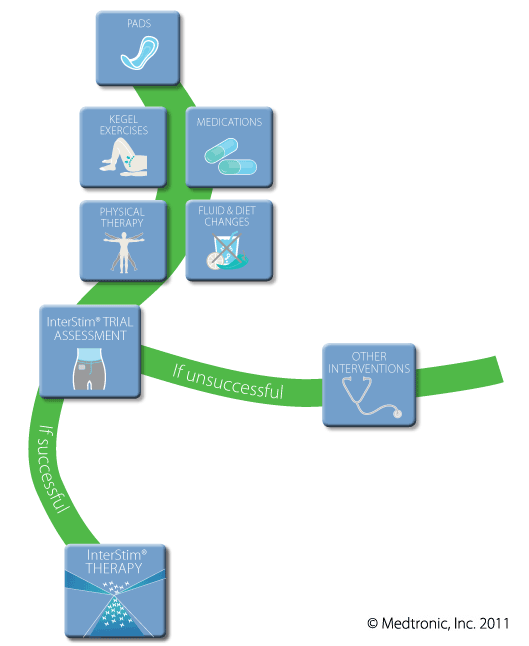
- If you achieve good results from the trial, you and your doctor can discuss long-term InterStim Therapy
Procedure
This is a two-stage procedure. We have staffed urologists that perform this procedure and will perform the first stage utilizing the staff and facilities at the Urology Associates of Central California Ambulatory Surgery Center. This stage consists of testing the patient and the device under careful observation for efficacy and tolerance.
Once it has been determined that the InterStim® Therapy is effective for the patient’s particular condition, the patient will be scheduled in a local hospital or at UACC for the complete implantation of the InterStim® device.
Voiding Diary
The voiding diary is used to track your bladder control symptoms for a few days. It will provide your doctor with valuable information.
You will be asked to:
- Record information, such as how often and how much you urinate or experience symptoms before test stimulation.
- Record how stimulation affects your symptoms during test stimulation.
- Monitor any return of symptoms after test stimulation.
You may need to complete several diaries before, during, and after the test stimulation. It is important to complete the diary accurately in order to give your doctor a detailed picture of how the therapy works for you.
The diary is small enough to tuck into a purse or pocket so that you can carry it with you. Your doctor will instruct you further in the use of the diary.
Why it’s Done.
InterStim® Therapy can eliminate or greatly reduce urinary symptoms for many people suffering from retention, urgency, frequency, and/or incontinence.
For instance, doctors have found that nearly half of patients with urge incontinence who received InterStim® Therapy were completely dry within six months of receiving the therapy. Many patients have their symptoms reduced significantly.n Americans deal with OAB1—defined as urgency-frequency and
|
Urge incontinence
- Do you lose urine the moment you realize you have to go to the bathroom?
- Do you leak urine when you drink even a small amount of liquid, when you hear or touch running water, or for no reason at all?
Urgency-frequency
- Do you have frequent, uncontrollable urges to urinatem more than seven times a day?
- Do you get up to use the bathroom more than once at night?
Urinary retention
- Do you have trouble emptying your bladder?
- Do you produce only a weak, dribbling stream of urine, leak urine, or not feel when your bladder is full?
Risks
InterStim Therapy is not intended for patients with a urinary blockage. Safety and effectiveness have not been established for pregnancy and delivery; patients under the age of 16; or for patients with neurological diseases such as multiple sclerosis.
In addition to risks related to surgery, complications can include pain at the implant sites, new pain, infection, lead (thin wire), movement/migration, device problems, interactions with certain other devices or diagnostic equipment, undesirable changes in urinary or bowel function, and uncomfortable stimulation (sometimes described as a jolting or shocking feeling).
Common questions about the Trial Assessment
Q: Will my everyday activities be affected during the trial assessment?
You should take it easy during the trial assessment period. Avoid bending, stretching, or lifting heavy objects. You can usually continue to work throughout your trial assessment if your job doesn’t require strenuous movement. Be aware that the trial assessment wire can move. Keep your activity level low to moderate.
Q: Will the trial assessment cure my condition?
No. The trial assessment is temporary. It is a tool that helps determine whether InterStim Therapy is appropriate for you. Once the flexible wire is removed, your original symptoms will return. If you have had positive results with the trial assessment, then you and your doctor may decide to use long-term InterStim Therapy to treat your symptoms.
Q: What does the stimulation feel like?
Stimulation varies from person to person, but most people describe it as a slight “pulling” or a “tingling” sensation in the pelvic area. It should not be painful. Talk to your doctor if you have any concerns.
Q: If my trial assessment is successful, what is long-term InterStim Therapy?
Long-term InterStim Therapy is a neurostimulator implanted during an outpatient procedure.
Q: Will insurance cover the cost?
Medicare in all 50 states and Puerto Rico, as well as many private insurance companies, covers InterStim Therapy.

 Imagine Hope
Imagine Hope